「Trichinellosis (Trichinosis)」の版間の差分
Vaccipedia.admin (トーク | 投稿記録) |
Vaccipedia.admin (トーク | 投稿記録) |
||
| (同じ利用者による、間の42版が非表示) | |||
| 1行目: | 1行目: | ||
{{TM menu}} | {{TM menu}} | ||
| + | ==Learning resources== | ||
*旋毛虫 in Japanese | *旋毛虫 in Japanese | ||
| − | ==Pathogen== | + | *[https://www.trichinella.org/home THE TRICHINELLA PAGE] |
| − | *''Trichinella | + | *[https://www.cdc.gov/dpdx/trichinellosis/index.html Trichinellosis - DPDx by US CDC] |
| − | **''Trichinella | + | *[http://www.ichiryusha.com/book/index.php?main_page=product_info&cPath=23&products_id=1154 Illustrated ''Trichinella'' written by Yuzo Takahashi] (not for sale) |
| − | + | ||
| − | + | ==Pathogen and Taxonomy== | |
| + | *The genus ''Trichinella'' has '''genetically distinguished''' but '''taxonomically still undetermined''' genotypes other than usual species | ||
| + | *The biggest morphological classification is based on the presence/absence of collagen capsule surrounding the pathogen in cysts in infected muscles | ||
| + | |||
| + | {|class="wikitable" | ||
| + | |- | ||
| + | !style="width:50%"|Encapsulated | ||
| + | !style="width:50%"|Non-encapsulated | ||
| + | |-style="text-align:center" | ||
| + | |Infect only mammals | ||
| + | |Infect birds and mammals | ||
| + | |-style="vertical-align:top" | ||
| + | | | ||
| + | *''Trichinella spiralis'' | ||
| + | *''Trichinella nativa'' | ||
| + | *''Trichinella nelsoni'' | ||
*''Trichinella britovi'' | *''Trichinella britovi'' | ||
| + | *''Trichinella murrelli'' | ||
| + | *''Trichinella patagoniensis'' | ||
| + | *''Trichinella'' genotype T6 | ||
| + | *''Trichinella'' genotype T8 | ||
| + | *''Trichinella'' genotype T9 | ||
| + | | | ||
*''Trichinella pseudospiralis'' | *''Trichinella pseudospiralis'' | ||
| + | *''Trichinella papuae'' | ||
| + | *''Trichinella zimbabwensis'' | ||
| + | |} | ||
| − | + | ※Manson's Tropical Infectious Diseases 24th ed. (published in 2023) describes that ''T. spiralis'' has several subspecies but according to [https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Undef&id=6333&lvl=3&keep=1&srchmode=1&unlock '''NCBI Taxonomy Browser'''] and the following articles subspecies written in Manson's are classified as species. | |
| − | + | ||
| + | {{quote|content= | ||
| + | Pozio, E., Rosa, G. la, Murrell, K. D., & Lichtenfels, J. R. (1992). Taxonomic Revision of the Genus Trichinella. The Journal of Parasitology, 78(4), 654. https://doi.org/10.2307/3283540 | ||
| + | }} | ||
| + | |||
| + | {{quote|content= | ||
| + | Zarlenga, D., Thompson, P., & Pozio, E. (2020). Trichinella species and genotypes. Research in Veterinary Science, 133, 289–296. https://doi.org/10.1016/j.rvsc.2020.08.012 | ||
| + | }} | ||
==Epidemiology== | ==Epidemiology== | ||
| + | *Since ''Trichinella'' infections often cause asymptomatic or mild disease and no serological tests with high performance is available, true epidemiology of human trichinellosis is thought still underestimated. | ||
| + | *Trichinellosis distributes '''worldwide''' from '''arctic region''' through '''the tropics'''. | ||
| + | *Human trichinellosis in developed countries has been dramatically decreased due to improvement of farming and slaughtering of domestic pigs and shrinkage of backyard pig farming in private facilities. | ||
| + | |||
| + | {{quote|content= | ||
| + | Yayeh, M., Yadesa, G., Erara, M., Fantahun, S., Gebru, A., & Birhan, M. (2020). Epidemiology, diagnosis and public health importance of Trichinellosis. Journal of World’s Poultry Research, 10(3), 131–139. https://doi.org/10.36380/scil.2020.ojafr18 | ||
| + | }} | ||
| + | |||
| + | *Distribution of species (directly linked from [https://www.trichinella.org/home THE TRICHINELLA PAGE]) | ||
| + | |||
| + | https://images.squarespace-cdn.com/content/v1/5adf9528f93fd46f6d3ddb95/1533145049330-8G0LCIZZ85UXA9J9CYP2/geodistribution.gif | ||
| + | |||
| + | ==Life cycle and Transmission== | ||
| + | *Life cycle is maintained amongst host mammals and birds. | ||
| + | **Pigs and rats (domestic cycle) or wild bores, wild bears, polar bears, rats and birds (sylvatic cycle). | ||
| + | **Humans are '''accidental (deadend) hosts''' for ''Trichinella'' | ||
| + | **'''Only humans develop clinical symptoms by ''Trichinella'' infection''' | ||
| + | *Refer to [https://www.cdc.gov/dpdx/trichinellosis/index.html DPDx - Trichinellosis] | ||
| + | https://www.cdc.gov/dpdx/trichinellosis/modules/Trichinella_LifeCycle.gif | ||
| + | |||
| + | *Transmission to human occurs by ingestion of raw or undercooked meat including '''pigs''', '''wild bores''', '''horse''', '''dog''', '''bear''', '''polar bear''', '''badger''' and '''soft-shelled turtle (スッポン)'''. | ||
| + | *Transmission to horse (obligate grazer) is speculated that pasture or hay may be accidentally contaminated by infected carcass (rodents etc.). | ||
| + | |||
| + | {{quote|content= | ||
| + | Rostami, A., Gamble, H. R., Dupouy-Camet, J., Khazan, H., & Bruschi, F. (2017). Meat sources of infection for outbreaks of human trichinellosis. Food Microbiology, 64, 65–71. https://doi.org/10.1016/j.fm.2016.12.012 | ||
| + | }} | ||
| − | + | *'''The world-first report''' of trichinellosis originated from '''soft-shelled turtle''' was published in Japan in 2009 (but only in Japanese and neglected from English literature). | |
| + | *The transmission route is speculated that soft-shelled turtles were fed by carrions of pigs dead by diseases and contaminated through the carrions. | ||
| − | == | + | {{quote|content= |
| + | 前田卓哉, 藤井毅, 岩本愛吉, 長野功, 呉志良, & 高橋優三. (2009). スッポンを感染源とする旋毛虫症例. 病原微生物検出情報, 30(10), 272–273. https://idsc.niid.go.jp/iasr/30/356/kj3563.html | ||
| + | }} | ||
| + | |||
| + | *Report of trichinellosis outbreak through game bear meat in Japan. | ||
| + | {{quote|content= | ||
| + | 海野友梨, 中本有美, & 深谷節子. (2017). 茨城県内で発生した旋毛虫による食中毒事例について. 茨城衛生研究所年報, 55, 37–41. https://www.pref.ibaraki.jp/hokenfukushi/eiken/kikaku/annualreport/documents/32_senmoutyu.pdf | ||
| + | }} | ||
| + | |||
| + | ==Human disease== | ||
| + | *Humans are '''accidental (deadend) hosts'''. | ||
| + | #Ingestion of larvae-infected meat | ||
| + | #'''Enteric phase''' | ||
| + | ##In 2-7 days incubation, larvae penetrate duodenal and jejunal mucosa | ||
| + | ##Nausea, vomitting, abdominal colic, fever | ||
| + | ###Maculopapular skin rash and pneumonitis may accompany | ||
| + | #'''Migration (invasion) phase''' | ||
| + | ##Larvae invade blood vessels and migrate toward striated muscle cells in '''diaphragm''', '''masseters''', '''intercostals''', '''laryngeal''', '''tongue''' and '''ocular muscles''' | ||
| + | ##Severe myalgia, difficulty of mastication, difficulty of breathing, dysphagia, periorbital edema, paralysis of extremities, high fever, petechiae in nails and conjunctivae | ||
| + | ##Eosinophilia arises but subsides in a week | ||
| + | ###In some case myocardial complication, neurological complication occurs | ||
| + | #'''Encystment phase''' | ||
| + | ##Weeks after infection, larvae encyst in striated muscles they arrived | ||
| + | ##Cachexia, edema, extreme dehydration | ||
| + | ##In 6 months calcification of cysts takes place | ||
| + | ##Inside calcified cysts, '''<nowiki>'</nowiki>nurse cells<nowiki>'</nowiki>''' which is transformed from normal striated muscle cells by larvae secretion encapsulate and nourish larvae | ||
| + | ##'''Encapsulated larvae can survive months to decades in human striated muscles''' | ||
| + | *The larger number of larvae infect, the more severe symptoms are | ||
| + | **<10 larvae: asymptomatic to mild | ||
| + | **50-500 larvae: moderate | ||
| + | **<1000 larvae: severe to fatal | ||
| + | |||
| + | ===Nurse cell=== | ||
| + | *Refer to [https://www.trichinella.org/the-nurse-cell Nurse cell formation in THE TRICHINELLA PAGE] or the following article | ||
| + | {{quote|content= | ||
| + | Wu, Z., Sofronic-Milosavljevic, L., Nagano, I., & Takahashi, Y. (2008). Trichinella spiralis: nurse cell formation with emphasis on analogy to muscle cell repair. Parasites & Vectors, 1(1), 27. https://doi.org/10.1186/1756-3305-1-27 | ||
| + | }} | ||
==Diagnosis== | ==Diagnosis== | ||
| + | {|class="wikitable" style="width:800px" | ||
| + | |+Case definitions by ECDC | ||
| + | |- | ||
| + | !style="width:33%"|Clinical | ||
| + | !style="width:33%"|Laboratory | ||
| + | !style="width:33%"|Epidemiological | ||
| + | |-style="vertical-align:top" | ||
| + | |At least 3 of | ||
| + | *Fever | ||
| + | *Myalgia | ||
| + | *Gastrointestinal symptoms | ||
| + | *Facial edema | ||
| + | *Eosinophillia | ||
| + | *Subconjunctival, sublingual and retinal hemorrhage | ||
| + | |At least 1 of | ||
| + | *''Trichinella'' larvae in muscle biopsy specimen | ||
| + | *''Trichinella''-specific antibody by ELISA or Western blot | ||
| + | |At least 1 of | ||
| + | *Ingestion of laboratory-confirmed contaminated meat | ||
| + | *Ingestion of potentially contaminated meat from laboratory-confirmed infected animal | ||
| + | *Epidemiological link to laboratory-confirmed human case with the common source | ||
| + | |} | ||
| + | {{quote|content= | ||
| + | Gottstein, B., Pozio, E., & Nöckler, K. (2009). Epidemiology, Diagnosis, Treatment, and Control of Trichinellosis. Clinical Microbiology Reviews, 22(1), 127–145. https://doi.org/10.1128/CMR.00026-08 | ||
| + | }} | ||
| + | |||
| + | |||
| + | *'''Trichinoscopy''' | ||
| + | **Encystment phase begins 1 week after infection at the shortest | ||
| + | **Muscle biopsy specimen is thin-sliced and pressed between two slides without any stain and cysts are observed | ||
| + | *ELISA for antibody detection is common | ||
| + | *Multiplex PCR is also used | ||
| + | |||
| + | ===Differential diagnoses=== | ||
| + | *Trichinellosis is a great mimicker | ||
| + | **Typhoid, encephalitis, myositis, tetanus, Katayama syndrome, hookworm infection, strongyloidiasis, periarthritis nodosa, rheumatoid arthritis | ||
==Treatment== | ==Treatment== | ||
| + | *Albendazole and mebendazole | ||
| + | |||
| + | ==Prevention and Control== | ||
| + | {|class="wikitable" | ||
| + | |- | ||
| + | !style="width:50%"|Heat | ||
| + | !style="width:50%"|Freezing | ||
| + | |- | ||
| + | |rowspan="2"| | ||
| + | *Meat core temperature '''≥71°C '''for''' ≥1 min''' | ||
| + | **Color to gray, muscle fibers separated | ||
| + | | | ||
| + | Up to '''15cm thickness''' meat block | ||
| + | *'''≤-15°C '''for''' ≥3 weeks''' | ||
| + | |- | ||
| + | | | ||
| + | Up to '''50cm thickness''' meat block | ||
| + | *'''≤-15°C '''for''' ≥4 weeks''' | ||
| + | |- | ||
| + | | | ||
| + | |※'''Applicable only to ''T. spiralis'' in pork''' | ||
| + | |} | ||
| + | |||
| + | ※Other species are more resistant to cold temperature | ||
| + | *''T. britovi'' in pork survived for 3 weeks at -20°C | ||
| + | *''T. spiralis'' in horse survived for 4 weeks at -18°C | ||
| + | *Game meat like bear harbors freeze-resistant ''Trichinella'' | ||
| + | **5 years in bear meat | ||
2023年9月23日 (土) 10:42時点における最新版
| Navigation Menu |
| General issues of Vaccine | |||||||
|---|---|---|---|---|---|---|---|
|
| General issues of Travel med. | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| Immunology | |||||||
|---|---|---|---|---|---|---|---|
|
| Epi & Stats | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| Virus | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| COVID-19 | |||||
|---|---|---|---|---|---|
|
| Bacteria | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| Rickettsia | |||||
|---|---|---|---|---|---|
|
| Protozoa | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| Fungi | |||||||
|---|---|---|---|---|---|---|---|
|
| Nematode (roundworm) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| Trematode (fluke, distoma) | |||||||
|---|---|---|---|---|---|---|---|
|
| Cestode (tapeworm) | ||||||
|---|---|---|---|---|---|---|
|
| Medical Zoology | ||||||||
|---|---|---|---|---|---|---|---|---|
|
目次
Learning resources
- 旋毛虫 in Japanese
- THE TRICHINELLA PAGE
- Trichinellosis - DPDx by US CDC
- Illustrated Trichinella written by Yuzo Takahashi (not for sale)
Pathogen and Taxonomy
- The genus Trichinella has genetically distinguished but taxonomically still undetermined genotypes other than usual species
- The biggest morphological classification is based on the presence/absence of collagen capsule surrounding the pathogen in cysts in infected muscles
| Encapsulated | Non-encapsulated |
|---|---|
| Infect only mammals | Infect birds and mammals |
|
|
※Manson's Tropical Infectious Diseases 24th ed. (published in 2023) describes that T. spiralis has several subspecies but according to NCBI Taxonomy Browser and the following articles subspecies written in Manson's are classified as species.
|
Pozio, E., Rosa, G. la, Murrell, K. D., & Lichtenfels, J. R. (1992). Taxonomic Revision of the Genus Trichinella. The Journal of Parasitology, 78(4), 654. https://doi.org/10.2307/3283540 |
|
Zarlenga, D., Thompson, P., & Pozio, E. (2020). Trichinella species and genotypes. Research in Veterinary Science, 133, 289–296. https://doi.org/10.1016/j.rvsc.2020.08.012 |
Epidemiology
- Since Trichinella infections often cause asymptomatic or mild disease and no serological tests with high performance is available, true epidemiology of human trichinellosis is thought still underestimated.
- Trichinellosis distributes worldwide from arctic region through the tropics.
- Human trichinellosis in developed countries has been dramatically decreased due to improvement of farming and slaughtering of domestic pigs and shrinkage of backyard pig farming in private facilities.
|
Yayeh, M., Yadesa, G., Erara, M., Fantahun, S., Gebru, A., & Birhan, M. (2020). Epidemiology, diagnosis and public health importance of Trichinellosis. Journal of World’s Poultry Research, 10(3), 131–139. https://doi.org/10.36380/scil.2020.ojafr18 |
- Distribution of species (directly linked from THE TRICHINELLA PAGE)
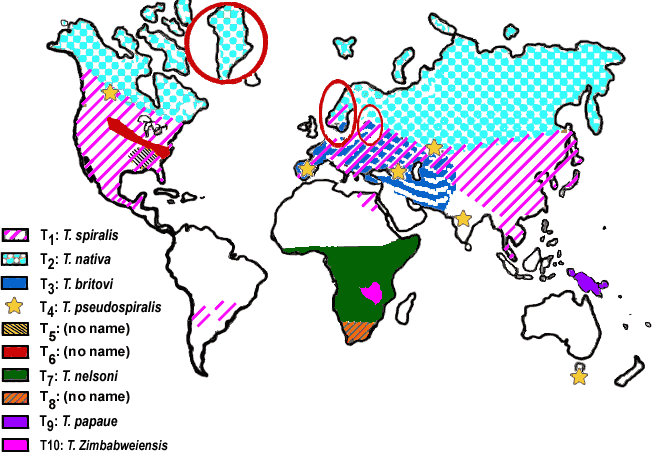
Life cycle and Transmission
- Life cycle is maintained amongst host mammals and birds.
- Pigs and rats (domestic cycle) or wild bores, wild bears, polar bears, rats and birds (sylvatic cycle).
- Humans are accidental (deadend) hosts for Trichinella
- Only humans develop clinical symptoms by Trichinella infection
- Refer to DPDx - Trichinellosis
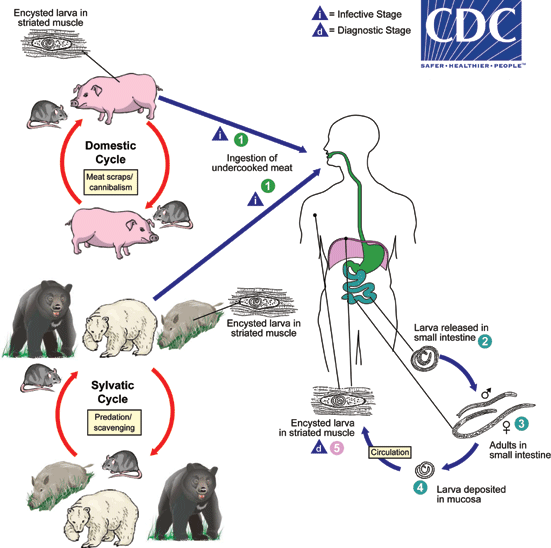
- Transmission to human occurs by ingestion of raw or undercooked meat including pigs, wild bores, horse, dog, bear, polar bear, badger and soft-shelled turtle (スッポン).
- Transmission to horse (obligate grazer) is speculated that pasture or hay may be accidentally contaminated by infected carcass (rodents etc.).
|
Rostami, A., Gamble, H. R., Dupouy-Camet, J., Khazan, H., & Bruschi, F. (2017). Meat sources of infection for outbreaks of human trichinellosis. Food Microbiology, 64, 65–71. https://doi.org/10.1016/j.fm.2016.12.012 |
- The world-first report of trichinellosis originated from soft-shelled turtle was published in Japan in 2009 (but only in Japanese and neglected from English literature).
- The transmission route is speculated that soft-shelled turtles were fed by carrions of pigs dead by diseases and contaminated through the carrions.
|
前田卓哉, 藤井毅, 岩本愛吉, 長野功, 呉志良, & 高橋優三. (2009). スッポンを感染源とする旋毛虫症例. 病原微生物検出情報, 30(10), 272–273. https://idsc.niid.go.jp/iasr/30/356/kj3563.html |
- Report of trichinellosis outbreak through game bear meat in Japan.
|
海野友梨, 中本有美, & 深谷節子. (2017). 茨城県内で発生した旋毛虫による食中毒事例について. 茨城衛生研究所年報, 55, 37–41. https://www.pref.ibaraki.jp/hokenfukushi/eiken/kikaku/annualreport/documents/32_senmoutyu.pdf |
Human disease
- Humans are accidental (deadend) hosts.
- Ingestion of larvae-infected meat
- Enteric phase
- In 2-7 days incubation, larvae penetrate duodenal and jejunal mucosa
- Nausea, vomitting, abdominal colic, fever
- Maculopapular skin rash and pneumonitis may accompany
- Migration (invasion) phase
- Larvae invade blood vessels and migrate toward striated muscle cells in diaphragm, masseters, intercostals, laryngeal, tongue and ocular muscles
- Severe myalgia, difficulty of mastication, difficulty of breathing, dysphagia, periorbital edema, paralysis of extremities, high fever, petechiae in nails and conjunctivae
- Eosinophilia arises but subsides in a week
- In some case myocardial complication, neurological complication occurs
- Encystment phase
- Weeks after infection, larvae encyst in striated muscles they arrived
- Cachexia, edema, extreme dehydration
- In 6 months calcification of cysts takes place
- Inside calcified cysts, 'nurse cells' which is transformed from normal striated muscle cells by larvae secretion encapsulate and nourish larvae
- Encapsulated larvae can survive months to decades in human striated muscles
- The larger number of larvae infect, the more severe symptoms are
- <10 larvae: asymptomatic to mild
- 50-500 larvae: moderate
- <1000 larvae: severe to fatal
Nurse cell
- Refer to Nurse cell formation in THE TRICHINELLA PAGE or the following article
|
Wu, Z., Sofronic-Milosavljevic, L., Nagano, I., & Takahashi, Y. (2008). Trichinella spiralis: nurse cell formation with emphasis on analogy to muscle cell repair. Parasites & Vectors, 1(1), 27. https://doi.org/10.1186/1756-3305-1-27 |
Diagnosis
| Clinical | Laboratory | Epidemiological |
|---|---|---|
At least 3 of
|
At least 1 of
|
At least 1 of
|
|
Gottstein, B., Pozio, E., & Nöckler, K. (2009). Epidemiology, Diagnosis, Treatment, and Control of Trichinellosis. Clinical Microbiology Reviews, 22(1), 127–145. https://doi.org/10.1128/CMR.00026-08 |
- Trichinoscopy
- Encystment phase begins 1 week after infection at the shortest
- Muscle biopsy specimen is thin-sliced and pressed between two slides without any stain and cysts are observed
- ELISA for antibody detection is common
- Multiplex PCR is also used
Differential diagnoses
- Trichinellosis is a great mimicker
- Typhoid, encephalitis, myositis, tetanus, Katayama syndrome, hookworm infection, strongyloidiasis, periarthritis nodosa, rheumatoid arthritis
Treatment
- Albendazole and mebendazole
Prevention and Control
| Heat | Freezing |
|---|---|
|
Up to 15cm thickness meat block
|
|
Up to 50cm thickness meat block
| |
| ※Applicable only to T. spiralis in pork |
※Other species are more resistant to cold temperature
- T. britovi in pork survived for 3 weeks at -20°C
- T. spiralis in horse survived for 4 weeks at -18°C
- Game meat like bear harbors freeze-resistant Trichinella
- 5 years in bear meat